Synthetic droplets cause a stir in the primordial soup
Our bodies are made up of trillions of different cells, each fulfilling their own unique function to keep us alive.
How do cells move around inside these extremely complicated systems? How do they know where to go? And how did they get so complicated to begin with? Simple yet profound questions like these are at the heart of curiosity-driven basic research, which focuses on the fundamental principles of natural phenomena. An important example is the process by which cells or organisms move in response to chemical signals in their environment, also known as chemotaxis.
A constellation of researchers from three different research units at the Okinawa Institute of Science and Technology (OIST) came together to answer basic questions about chemotaxis by creating synthetic droplets to mimic the phenomena in the lab, allowing them to precisely isolate, control and study the phenomena. Their results, which helps answering questions about the principles of movement in simple biological systems, have now been published in the Journal of The American Chemical Society. “We have shown that it is possible to make protein droplets migrate through simple chemical interactions,” says Alessandro Bevilacqua, PhD student in the Protein Engineering and Evolution Unit and co-first author on the paper. Professor Paola Laurino, head of the unit and leading author, adds that they “have created a simple system that mimic a very complex phenomenon, and which can be modulated through enzymatic activity.”
Tensions on the surface
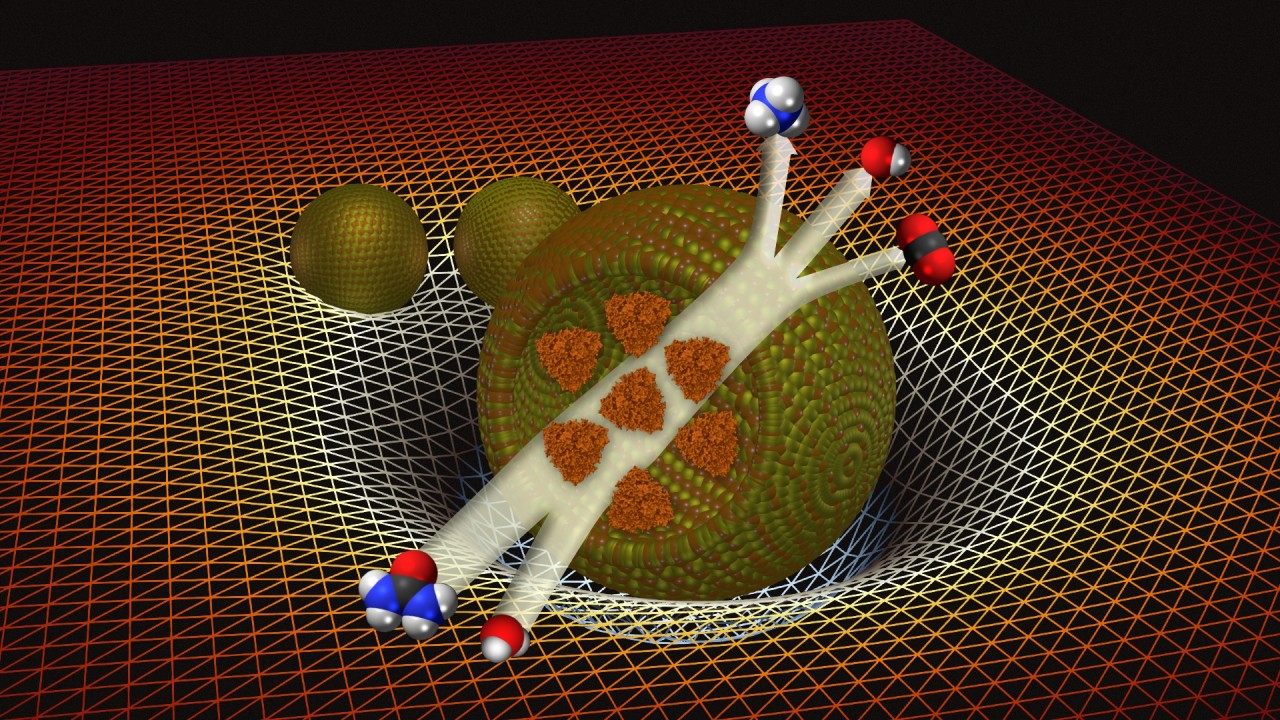
While the process of creating droplets might not sound like the most complicated task, mimicking biological processes as close to reality as possible while keeping accurate control over all the variables certainly is. The synthetic, membrane-less droplets contain a very high concentration of the bovine protein BSA to mimic the crowded conditions inside cells, as well as urease, an enzyme that catalyzes the breakdown of urea into ammonia.
Ammonia is basic, meaning it has a high pH-value. As the enzyme gradually catalyzes the production of ammonia, it diffuses into the solution, creating a ‘halo’ of higher pH around the droplet, which in turn enables droplets to detect other droplets and migrate towards each other.

The researchers found that the key to understanding the chemotaxis of the droplets is the pH-gradient, as it facilitates the Marangoni effect, which describes how molecules flow from areas of high surface tension to low. Surface tension is the measure of energy required to keep molecules at the surface together, like glue. When pH increases, this glue weakens, causing molecules to spread out and lowering surface tension, which in turn makes it easier for molecules to move. You can see this by adding soap, which has a high pH, to one end of a bathtub of still water: the water will flow towards the end with soap because of the Marangoni effect.
When two synthetic droplets are close enough, their halos interact, raising the pH in the environment between them, which makes them move together. Because the surface tension is still strong on the opposite ends of the droplets, they keep their shape until the surfaces touch, and the cohesive forces within the droplets overcome the surface tension, causing them to merge. As larger droplets both produce more ammonia and have a larger surface area (which decreases surface tension), they attract droplets smaller than themselves.
Collaborating on ancient soup and future biotech
Thanks to the development of these droplets, the researchers have made headway in answering basic questions about biological movement – and in doing so, they have gained insight into the directed movement of the earliest forms of life in the primordial soup billions of years ago, as well as a lead on creating new biologically inspired materials.
Our knowledge of life as it looked billions of years ago is fuzzy at best. A prominent hypothesis is that life originated in the oceans, as organic molecules gradually assembled and became more sophisticated in a ‘primordial soup’ – and this could have been facilitated by chemotaxis through the Marangoni effect. “It would have been beneficial for droplets to have this mechanism of migration in the hypothetical origin of life scenario,” as Professor Laurino puts it. This migration could have triggered the formation of primitive metabolic pathways whereby enzymes catalyze a variety of substances that ultimately produce a chemical gradient that drives the droplets together, leading to larger and more sophisticated communities.
The research also points ahead in time, providing leads on new technology. “One example is the creation of responsive materials inspired by biology,” suggests Alessandro Bevilacqua. “We have shown how simple droplets can migrate thanks to a chemical gradient. A future application of this could be technologies that sense or react to chemical gradients, for example in micro-robotics or drug delivery.”
The work to produce and analyze the synthetic droplets is the result of a combination of deeply integrated interdisciplinarity and the human factors undergirding scientific work. The project began during the coronavirus pandemic, when a member of the Protein Engineering and Evolution Unit was in quarantine with a member of the Complex Fluids and Flows Unit. The two began talking, and though the two units are from two disparate fields – biochemistry and mechanics, respectively – the project evolved in tandem. Eventually, members from the Micro/Bio/Nanofluidics Unit joined the project with sophisticated measurements of the droplets’ surface tension.
The unique non-disciplinary research environment at OIST catalyzed the collaboration. As Professor Laurino puts it, “this project could never have existed if we were separated by departments. It hasn’t been an easy collaboration, because we communicate our field in very different ways – but being physically close made it significantly easier.” Alessandro Bevilacqua joins in: “The coffee factor has been very important. Being able to sit down with other unit members made the process much faster and more productive.” Their cooperation doesn’t stop here – rather, this paper is the beginning of a fruitful partnership between the three units. “We see a lot of synergy in our work, and we work effectively and efficiently together. I don’t see a reason why we should stop,” as Professor Laurino states it. It’s thanks to the combined efforts of the three units that we now know more about the minute movements of life at the smallest, earliest, and possibly future scale.

Article Information
Specialties
For press enquiries:
Press Inquiry Form