Cytoskeletal proteins play maintenance roles in neurotransmission
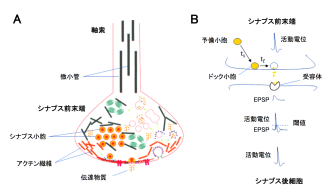
Researchers in the Cellular and Molecular Synaptic Function Unit at the Okinawa Institute of Science and Technology Graduate University (OIST) have elucidated the roles of cytoskeletal proteins at the giant presynaptic terminal, called the calyx of Held, visualized in rat brainstem slices. Initial findings were made by a graduate school student Lashmi Pyriya for her PhD thesis work under technical instructions by Dr Kohgaku Eguchi (now in IST Austria) and Dr Laurent Guillaud. This work was then substantiated by Dr Han-Ying Wang (Fig 3) and finally accepted for publication in the Journal of Neuroscience.
The scientists’ findings provide crucial insight into the function and location of microtubules in neurotransmission. Their research may also shed light on mechanisms of synaptic dysfunctions occurring in neuronal diseases like Alzheimer’s Disease and Parkinson’s Disease.

The main findings are 3-fold.
(1) Co-existence of MTs with SVs in presynaptic terminals
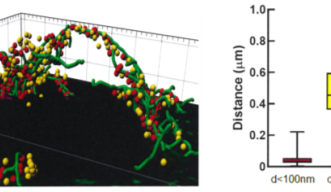
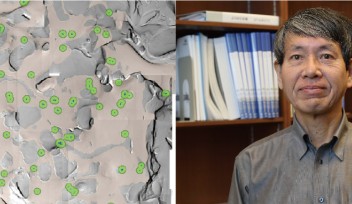
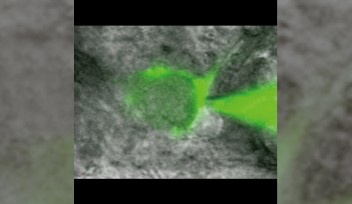
It has been controversial whether MTs exist in presynaptic terminals, and none of past reports support co-existence of MTs with SVs.Using a high-resolution STED microscope, at the calyx of Held, OIST researchers successfully demonstrated that ~50% of SVs co-exist with MTs with an average distance of 44 nm (Fig 2).

(2) F-actin and MTs support fast and slow SV replenishments, respectively
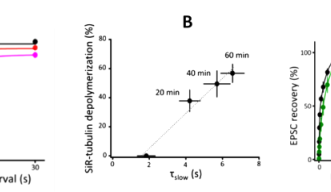
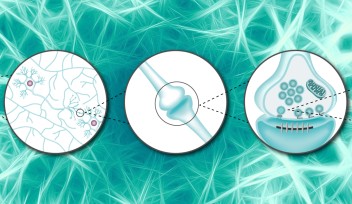
Soon after SVs are docked, they release transmitter at release sites, which is then immediately (tf 0.1s) replenished by docked SVs and docking site is then replenished more slowly (ts 2s) by reserve SVs (Fig 1B). When MTs were depolymerized, the slow component of SV replenishment prolonged (Fig 3A). The magnitude of prolongation was proportional to that of MT depolymerization assayed by live imaging (Fig 3B, correlation factor, 0.98). In contrast, F-actin depolymerization specifically prolonged the fast replenishing rate tf (Fig 3C).

(3) F-actin and MT support sustained high-frequency neurotransmission
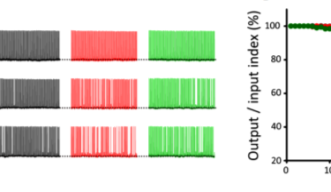
Delay of SV replenishment will impair maintenance of neurotransmission, particularly at high frequency. At the calyx of Held synapse, presynaptic stimulations at 100 Hz unfailingly generate APs in postsynaptic neurons for at least 50 s. When MTs are depolymerized, postsynaptic APs start to fail at around 20s and AP failures reach 40% after 50s stimulation. The effect of F-actin depolymerization is relatively weak, with 20% failures after 50s stimulation (Fig 4). These results indicate that cytoskeletal proteins in presynaptic terminals are essential for the maintenance of high-frequency neurotransmission.

These findings provide a new insight into the mechanisms of synaptic dysfunctions occurring in neuronal diseases. Since both a-synuclein in Parkinson’s disease and tau protein in Alzheimer disease exert toxic functions in presynaptic terminals, it is of crucial importance to elucidate presynaptic molecular mechanisms involved in the etiology of neuronal diseases.

Specialties
Research Unit
For press enquiries:
Press Inquiry Form