Basis of a Novel Drug-delivery System May be Hiding in our Cells
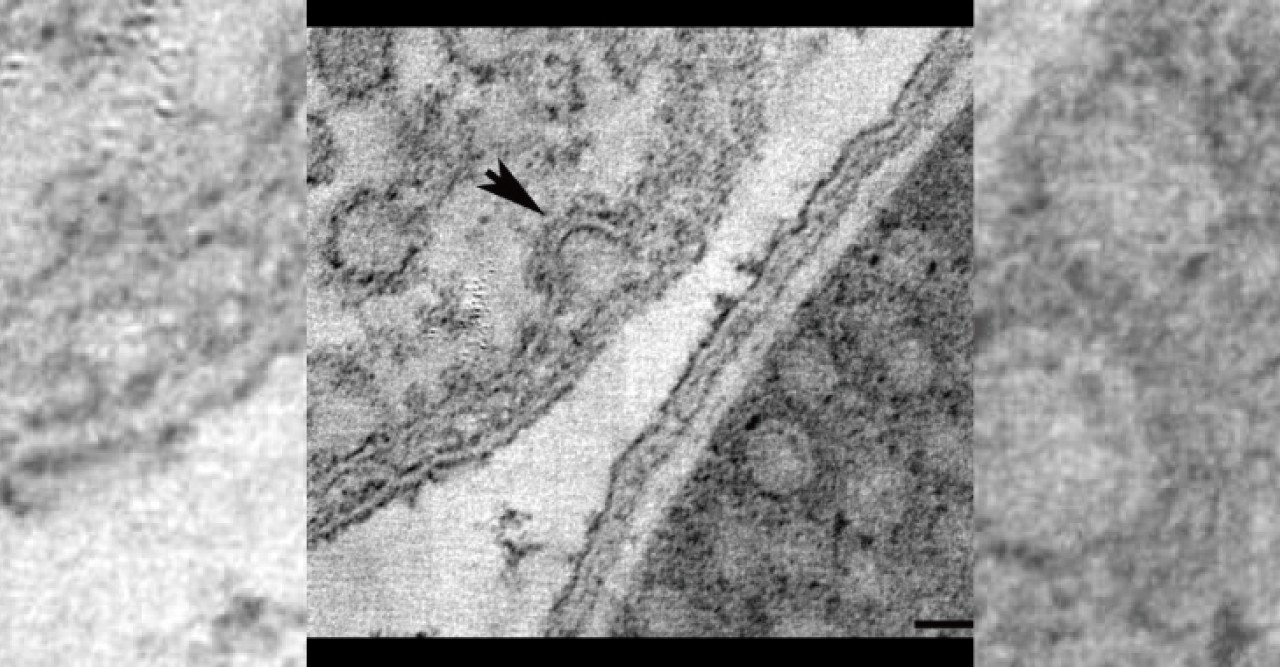
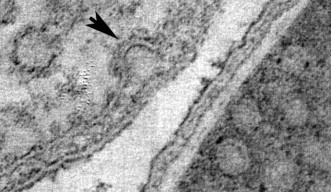
Sixty years ago, researchers looking at electron microscopy images found dense clusters of inlets budding from the cell membranes—the outer skins—of one type of cell. Dubbed caveolae, or “small caves,” by their discoverers, the structures were later found in similarly large numbers in some other types of cells, and turned out to be involved in ferrying new patches to the cell membrane, among other functions. But, because their chemical makeup made them difficult to work with, discerning exactly how caveolae come together, and how they function, has been slow going. In a paper published on August 17 in Cell, a team of researchers spanning three continents details a major step forward in understanding what caveolae look like and how they behave—and points the way toward a potentially game-changing method of drug delivery.
The team, led by Prof. Robert Parton of the University of Queensland in Australia and including researchers from OIST, India’s National Centre for Biological Sciences, the University Medical Center Utrecht in The Netherlands, and the Max Planck Institute for Molecular Cell Biology and Genetics in Germany, took several approaches to learning more about caveolae and the proteins that serve as their foundation, caveolins. Their approach was to put human caveolin genes into E. coli bacteria, which normally don’t have caveolae. The altered bacteria did indeed develop caveolae, confirming that caveolins are the crucial ingredient in the structures (the other main components are lipids and cholesterol, which the E. coli already had).
Parton's group in Queensland flash-froze the caveolae-containing cells and imaged them with a transmission electron microscope, then sent the data to OIST's Prof. Ulf Skoglund, who heads the Structural Cellular Biology Unit. Skoglund used the data to reconstruct three-dimensional images of the caveolae as they look in living organisms. He found that the E. coli caveola looked identical to the human version, meaning that the lab-generated caveola could be studied as proxies for the originals.
But because they could be made cheaply and in large quantities, the E. coli-generated capsules might have medical applications as well. In fact, they might be a way to achieve what has long been a holy grail in medicine: delivering drugs to the precise place in the body—a tumor, for example—where they are needed. To further explore this possibility, Parton’s group in Queensland tested whether the caveolae could be made with a protein embedded in their membranes that would bind only to a corresponding receptor on target cells. Like a key in a lock, the protein would trigger the target cell to let the caveola—and its contents—in. The group found that this did work in the lab, without causing any unintended effects for the target cells. Specially-engineered caveolae could theoretically be injected into a patient and float harmlessly through the bloodstream before reaching their target and delivering their payload of medicine.
Skoglund is optimistic about the discovery, but cautions: “This is still early, so we don’t know whether this is going to be the final delivery form.” His next step is to get a more precise picture of caveola structure. “We can see already that these caveolae have a pronounced polyhedral shape. We would like to further elucidate this shape, and hope to get higher-resolution images and analyze the way caveolins are organized in the caveolae,” he says.
Specialties
For press enquiries:
Press Inquiry Form