Shaping the Way to See the World
The proliferation of cells, in particular the orientation in which they divide, is key in regulating the shapes of tissues. However, the cellular mechanisms that govern cell proliferation and cell division orientation are not fully understood. Research conducted by Dr. Toshiaki Mochizuki, Dr. Shohei Suzuki and Prof. Ichiro Masai of the Developmental Neurobiology Unit at the Okinawa Institute of Science and Technology Graduate University, aimed to decipher these mechanisms by studying cell proliferation in the lenses of zebrafish. Their recent findings are being published in the October, 15th 2014 of Biology Open, appearing on the cover of the journal.
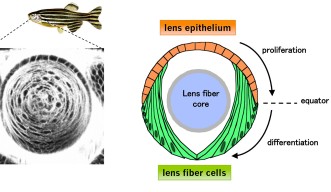
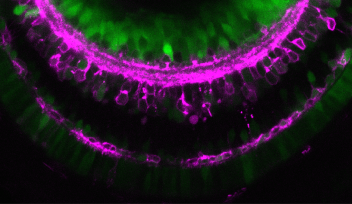
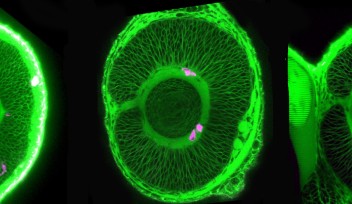
The lens is an organ within the eye, which is responsible for focusing incoming light onto the retina to form an image. Its size and shape are critical in order for it to focus the exact amounts of light needed to form an image, making the cells that comprise it a point of interest for researchers to know more about how cell proliferation contributes to lens growth. There are two types of cell, which make up the lens; lens epithelial cells, which cover the outer most surface of the front half of the lens in an area called the epithelium, and lens fiber cells which comprise the rest of the lens structure, including the lens fiber core.
Lens epithelial cells covering the front half of the lens begin to differentiate at the peripheral margins, or about half way around the lens. At this area, labeled the equator in Figure 4, epithelial cells become lens fiber cells, making this region the most active in terms of cell proliferation. But while previous studies have confirmed that these marginal zones are highly proliferating, the mechanisms that regulate proliferation of cells in this specific area remain unknown. The OIST researchers have looked at the cell cycle on the lens epithelium in more detail.
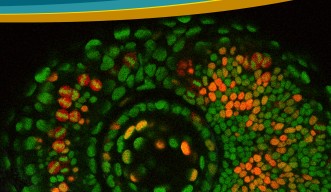
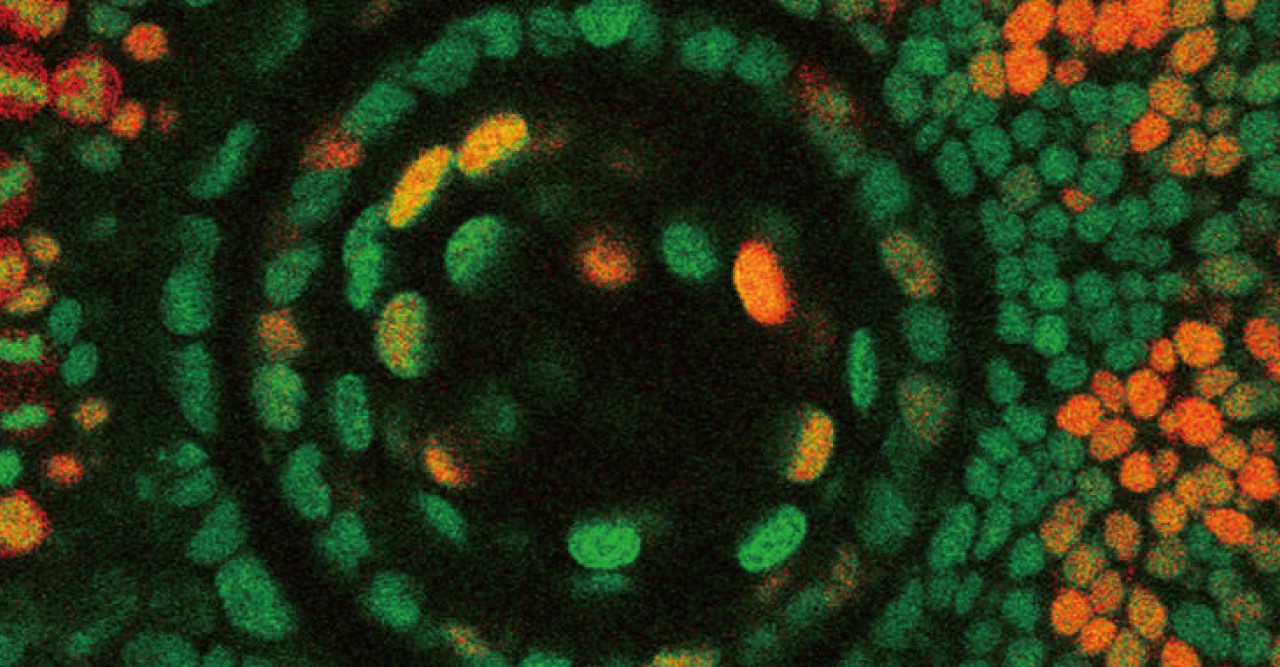
The cell cycle consists of four phases: G1, S, G2 and M. These phases, however, are difficult to tell apart simply by examining the morphology or outer structure of the cells. To better visualize cell proliferation, time lapse observation was carried out by the OIST researchers on the zebrafish lens epithelium. In order to accurately track which phase individual cells were at in the cell cycle, zebrafish transgenic lines were used. These transgenic fish contain fluorescent proteins, which are manifested during particular phases of the cell cycle. The fluorescent proteins are different colors, allowing researchers to see approximately which phase individual cells are at in the cell cycle and also to see which areas in the lens are more active in cell proliferation.
Their findings suggest that, like other vertebrates, cell proliferation is most active in the peripheral zone in zebrafish epithelium, and that the orientation of cell division is spatially patterned; meaning the orientation of cell division was dependent on the location in which the cell was dividing on the epithelium. Also the researchers found that a cell adhesion molecule E-cadherin influences epithelial cell shape, which subsequently regulates spatial pattern of cell-division orientation. Mochizuki explains “This work is a first description about cell division and cell morphology pattern in the lens epithelium, thus it may help to understand how the lens obtains and maintains its morphology. In addition, it might also help to understand eye diseases such as myopia and astigmatism.”
Prof. Masai elaborates on why time lapse observation of cell proliferation is important “Prior to studies like this, cell growth and division was mainly observed in a two dimensional state on petri dishes using cell culture samples. Such studies are not sufficient for answering questions about the role cell proliferation plays on the creation of a very precisely shaped three dimensional organ like the lens.” Observational techniques such as the one employed by OIST researchers can now reveal just how the lens takes shape during its development.
By Sean To
Specialties
Research Unit
For press enquiries:
Press Inquiry Form