The Mystery of Cell Proliferation: Matching Histone to DNA
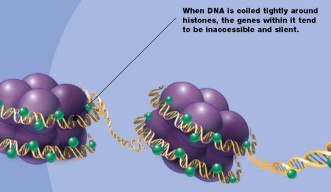
Before cells divide, they create so much genetic material that it must be wound onto spools before the two new cells can split apart. These spools are actually proteins called histones, and they must multiply at the same moment that the cell doubles its DNA. If the amount of histones does not increase when the DNA doubles, the centimeters of new DNA could never be packed small enough to fit into chromosomes, which are just a few micrometers long. In the early stage of development, the period when DNA doubles and the cell divides is called proliferation, after which an embryo grows from one cell to more than one thousand cells. Eventually, the new cells will differentiate into specific tissues. Even though cell proliferation is common in all tissue development, researchers do not fully understand how the amount of histone proteins increases in proportion to the amount of DNA during cell proliferation.
Professor Ichiro Masai, who heads the Okinawa Institute of Science and Technology Graduate University’s Developmental Neurobiology Unit, has spent years screening zebrafish with developmental mutations to understand cell division and differentiation. In this study, Masai and his lab identified one zebrafish strain carrying a mutation in the stem-loop binding protein, or SLBP. Previously, researchers identified SLBP as a key factor regulating the level of histones using in vitro studies, in cultured cells. Using zebrafish as a vertebrate animal model, Masai has shown that SLBP is necessary for development to function properly. Masai’s results, the first to show SLBP function in vivo, were published August 5, 2014 in Developmental Biology.
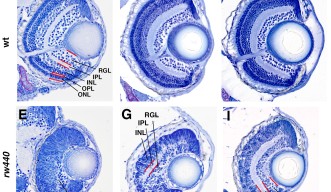
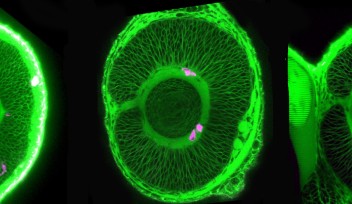
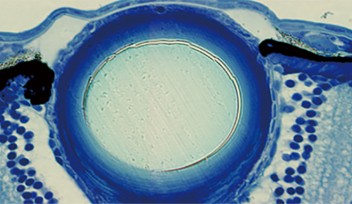
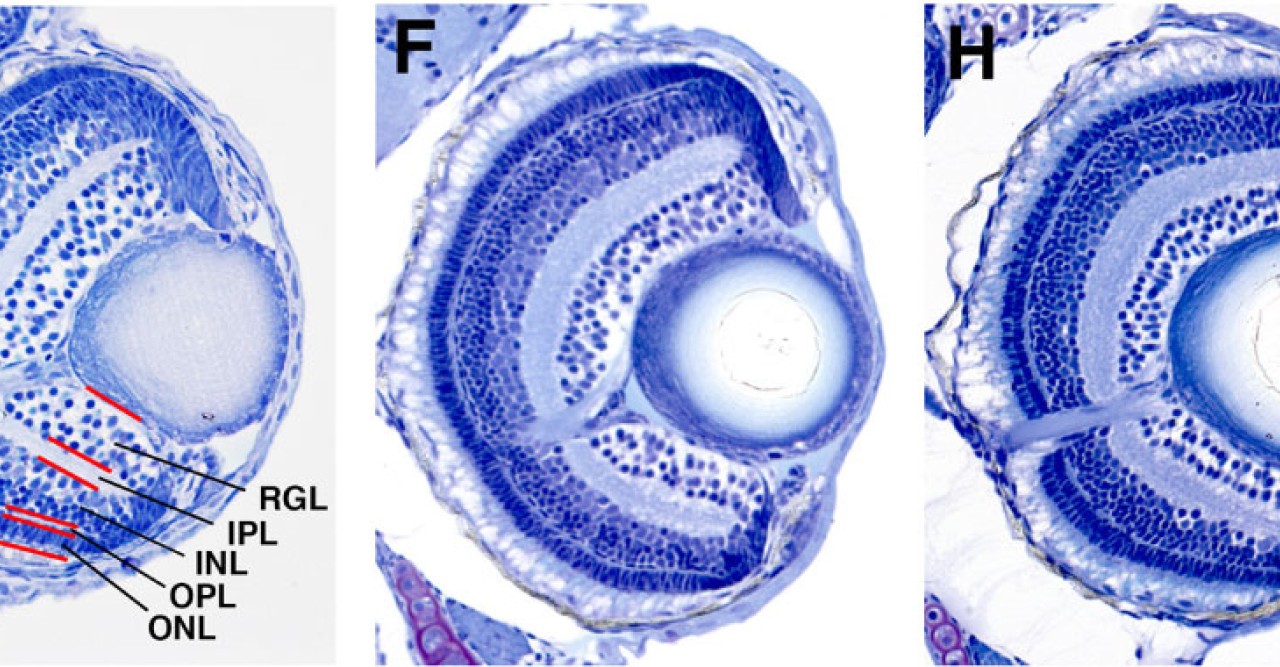
“I wanted to understand the cell differentiation process,” explained Masai. One might expect that a zebrafish without a fully functional SLBP gene would simply die, since the embryo would never be able to undergo cell division and pass the one-cell stage. But this is not the case; somehow the fish bypass the SLBP pathway, continuing proliferation so that the embryo can survive. Masai focused on the zebrafish’s retina to compare development between the mutant strain and the normally functioning, wild type strain. In the SLBP mutant embryos, retinal cells proliferate much more slowly than wild type embryos, and differentiate into fewer types of cells. The mutant embryos form less orderly layers of retinal tissue, making it difficult for signals to travel from light sensitive photoreceptors through the network of neurons in the retina. Finally, the mutant embryo’s retinal axon, which should carry visual signals from the retina to the brain, never exits the eye. Even if the mutant fish’s photoreceptors can interpret light, the light signals would never reach the brain.
Masai’s findings impact more than just a few zebrafish. Zebrafish SLBP is homologous to human SLBP, meaning that despite how distantly related humans and zebrafish are, the genetic sequences look very similar and the protein appears to function in the same way. “The development issues we see in zebrafish could be happening in humans,” said Masai, “but we would not know because we cannot take out a human eye to examine it.”
Furthermore, researchers have seen issues in cell division in many types of cancer. Specifically, the cell division issues occur during chromatin segregation, that mystery moment after the cell doubles its DNA and before it splits into two new cells. SLBP mutants in invertebrate animals showed defects in chromatin segregation, so Masai and his colleagues think that studying the protein in zebrafish could provide more insight.
Without a complete understanding of the genes regulating cell proliferation, division, and differentiation, no one can say exactly what goes awry in cancerous cells. Masai is taking the first steps to learn which genes are involved and how they work, one zebrafish at a time.
By Poncie Rutsch
Specialties
Research Unit
For press enquiries:
Press Inquiry Form