Sparking insights: stripping away the mysteries of our body’s electrical wires
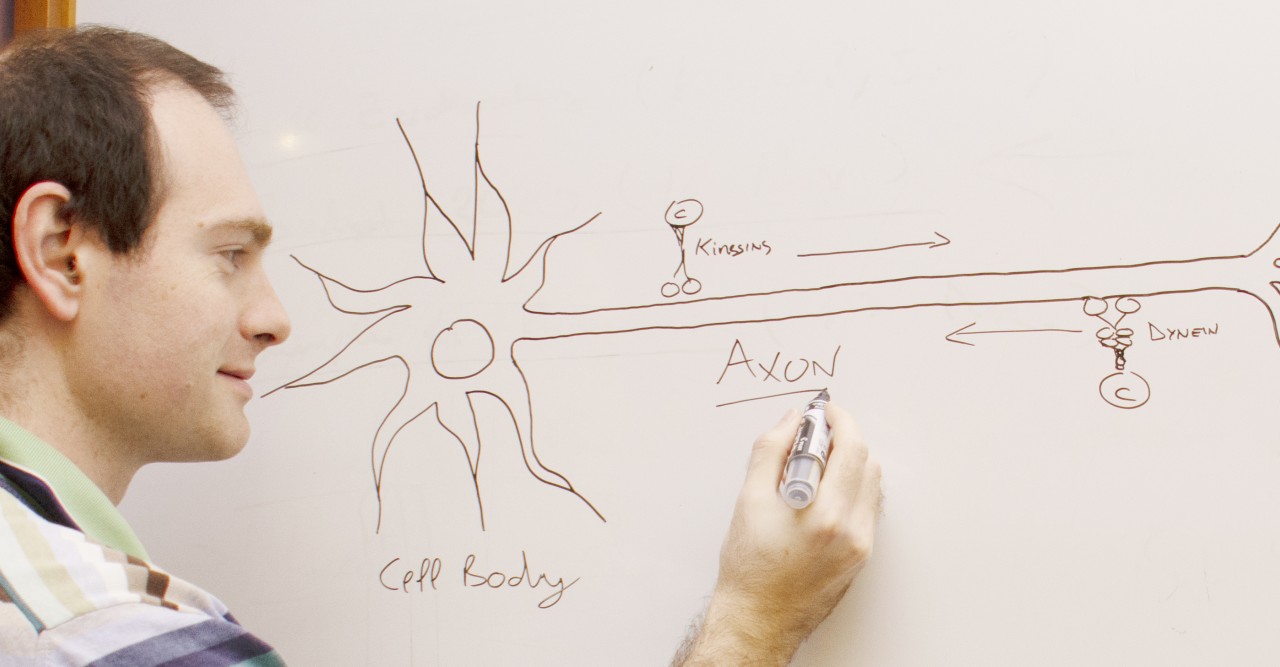
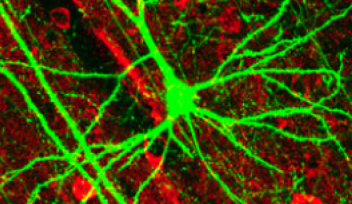
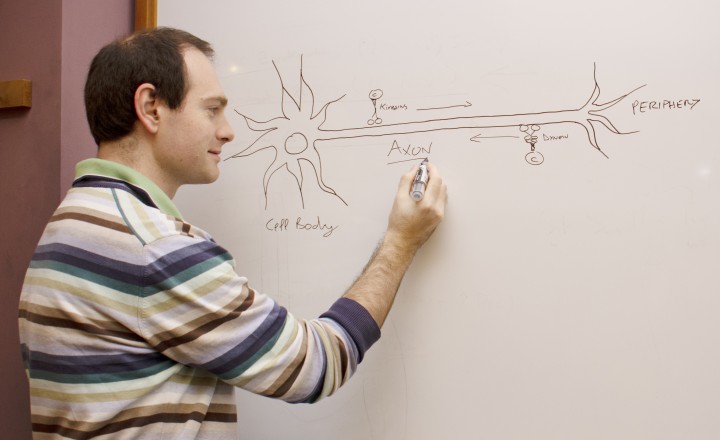
Neurons, the electrical wiring in our bodies that allows us to move, think and sense our surroundings, are specially adapted for rapid communication, highlighted by their unusual, elongated shape. The longest and thinnest part of the neuron is the axon, which conducts electrical signals away from the cell body to the neuron’s tips. But the axon also has a second function that is crucial for the health of neurons: it acts as the cell’s molecular motorway.
Professor Marco Terenzio, who leads the newly formed Molecular Neuroscience Unit at the Okinawa Institute of Science and Technology Graduate University (OIST), is unravelling the role that axons play in transport, through experiments using mouse models and neurons in culture.
“All sorts of cell components are shuttled along axons, including RNA, proteins, organelles and survival signals called neurotrophins,” said Prof. Terenzio. “Axons can be incredibly long – up to one meter in length in humans – so it’s quite remarkable how neurons are able to achieve this transport.”
Defects in transport along axons are one of the early warning signs of Parkinson’s, Alzheimer’s and other neurodegenerative diseases. Therefore, understanding how it occurs and identifying what is transported could ultimately lead to new methods for diagnosis and treatment of these pathologies, Prof. Terenzio said.
Loss of dynein subunit blocks the road
Prof. Terenzio joined OIST in October 2019, after doing postdoctoral research at the Weizmann Institute of Science on a protein called cytoplasmic dynein, which transports cargo in the retrograde direction, from the tips of neurons towards the cell body.
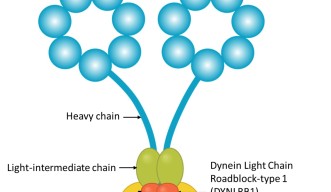
Dynein is a large and complex protein, composed of several subunits – or chains – which are classed by size. Working with his mentor at the Weizmann Institute, Professor Mike Fainzilber, he studied the role of a dynein subunit called Dynein Light Chain Roadblock-type 1 (DYNLRB1).
“The roles of the larger, heavy chains are fairly well understood, and diseases of the nervous system have been associated with mutated heavy chains, but the smaller, light chains, such as DYNLRB1, have so far been less studied,” Prof. Terenzio said.

In a study recently published in Neurobiology of Disease, Prof. Terenzio bred mice in which DYNLRB1 was selectively deleted in a certain type of sensory neurons called proprioceptive neurons, which are involved in balance and coordination. With the subunit missing, the number of proprioceptive neurons in the spinal nerve fell sharply. The mice also walked abnormally and had poor balance.
“Our results showed that DYNLRB1 is essential to the survival and maintenance of proprioceptive neurons,” Prof. Terenzio said. “This was really unexpected, as if you look at the enormous complexity of the dynein motor, you wouldn’t expect such a tiny subunit to make such an important difference.”
DYNLRB1 is believed to act as an accessory protein, linking dynein to cargo. Prof. Terenzio wanted to determine whether the subunit had such a damaging impact because it affected the function of the dynein protein as a whole, or whether it just affected the transport of certain substances. When Prof. Terenzio examined the neurons in cell culture, he found the latter to be true.
“This is really exciting as it means that whatever cargo is normally able to bind to dynein when DYNLRB1 is present is extremely important for maintaining the health of a sensory neuron – it is something that is absolutely required,” said Prof. Terenzio. “The next step forward is to find out what this cargo is.”
Now at OIST, the Molecular Neuroscience Unit is building on this research by developing a technique that tags any protein that moves close enough to DYNLRB1 to interact with it. They aim to use the technique to identify which proteins DYNLRB1 helps transport, which could potentially point to new signaling pathways necessary for neuron survival. The unit then plans to check whether these pathways are connected to known neurodegenerative diseases.

Found in translation – the secrets of survival and regeneration
Prof. Terenzio is also examining an axonal mechanism called local translation, which is thought to be important for neuronal survival. Cells normally synthesize proteins by translating a coded RNA sequence into a string of amino acids. That process is generally thought to occur near the nucleus but that’s not entirely true for neurons.
“Studies have shown that not all translation occurs in the cell body of neurons; RNA is instead transported along the axons and used to synthesize proteins where they are locally required, which could be more energetically efficient for large cells such as neurons,” explained Prof. Terenzio.
Prof. Terenzio is interested in looking at how local translation occurs in the context of neurodegenerative disease and after acute injury of the peripheral nervous system. This research involves collaborations with groups at Queen Mary, University of London, the Weizmann Institute of Science, Imperial College London and scientists inside Japan.
For one of these collaborations, the researchers hope to derive motor neurons from induced pluripotent stem cells reprogrammed from skin cells of people with Amyotrophic Lateral Sclerosis (ALS), a motor neuron disease that leads to paralysis and death. These patient-derived cells can reflect disease biology more accurately than mouse models, Prof. Terenzio said.
By comparing axonal translation in motor neurons in people with and without neurodegenerative disease, he hopes both to pinpoint the basic mechanisms of neuron survival and to develop a new method of screening for early signs of the disease.
The work on axonal translation also has applications for acute neuronal injury. “Axonal translation has an important role in stimulating the regeneration process after neurons are damaged. By studying the proteins synthesized, we would ultimately like to develop chemicals that can promote regeneration, potentially even in neurons that can’t naturally regenerate,” said Prof. Terenzio. “This would have enormous therapeutic potential for individuals with currently irreparable nerve damage.”
Specialties
Research Unit
For press enquiries:
Press Inquiry Form