A Step toward Clarification of the Mechanisms of Osteoporosis

In the bones supporting our bodies, calcium regulation occurs by balancing the activities of osteoclasts, which are groups of cells that increase the calcium concentration in blood by destroying bone tissue, and osteoblasts, which are another groups of cells that absorb calcium in blood to store in the bones. This process, called bone metabolism, is largely regulated by hormones, but more recently, the involvement of an intracellular protein named CNOT has been discovered. There are 11 known types of CNOTs. CNOTs are known for their role in mRNA degradation, in which CNOTs eliminate excess messenger RNA (mRNA) and inhibit overproduction of proteins in cells. Various studies have been undertaken to investigate whether a deficiency of each CNOT can cause disease.
At OIST, Prof. Tadashi Yamamoto and members of his Cell Signal Unit have been at the forefront of CNOT research. The team is investigating the physiological functions of the mammalian CNOTs and their mechanisms. Prof. Yamamoto first pointed out that a receptor protein, which receives extracellular signals, sends a message to CNOTs to regulate mRNA degradation. He also discovered that CNOT is involved in the regulation of gene expression. Now, his group is looking into the functions of each CNOT in human homeostasis – how the internal conditions of a human body maintain stability regardless of what is going on in the external environment. Specifically, their study focuses on the functions of a specific CNOT by analyzing mice in which a corresponding gene is removed.
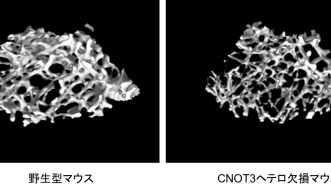
On February 4, a team led by Prof. Masaki Noda of Tokyo Medical and Dental University published a study in Proceedings of the National Academy of Sciences (PNAS) demonstrating that CNOT3 plays an important role in the pathogenesis of ageing-induced osteoporosis. Prof. Noda, a known expert in bone research, explored the relationship between CNOT3 and bone metabolism in collaboration with the OIST Cell Signal Unit. The researchers discovered that bones of mice whose CNOT3 expression was suppressed are fragile. They also revealed that CNOT3 stabilizes a specific mRNA which produces an osteoclast cell-surface receptor called RANK. CNOT3 deficiency, therefore, enhances production and activity of the RANK receptor, and stimulates osteoclasts, responsible for re-abosorbing bone tissue. The results suggest that a reduction of CNOT3 expression in bone tissue causes a decrease in bone density in the elderly, contributing to the development of osteoporosis.
In addition to exploring the overall functions of CNOTs at a molecular level, the OIST Cell Signal Unit has been offering other scientists their findings on these intracellular proteins, their expertise with gene-deficient mice, and their analytical expertise on CNOT. Prof. Yamamoto said, “By bringing together individual expertise, we can elucidate mechanisms of various biological phenomena. I am particularly eager to discover mechanisms behind the development of various diseases associated with CNOTs.”
By Mayumi Nishioka
Research Unit
For press enquiries:
Press Inquiry Form