From blood vessels as pathways to neurons as gatekeepers: Journey of immune cells in developing zebrafish revealed
Highlights
- Microglia, the immune cells of the brain, form the first line of defense against neurogenerative diseases and traumatic brain injuries.
- However, questions surrounding how the precursors of microglia travel to the different brain regions during development remain unanswered.
- Now, researchers have tracked these precursors in zebrafish embryos from 24 hours to 60 hours after fertilization to uncover how they travel to the developing retina.
- They found that the precursors use the surface of blood vessels as pathways to find the entrance of the retina and wait for the formation of neurons to begin before fully colonizing it.
- In the future, this knowledge could lead to targeted microglia stem cell therapies for neurodegenerative diseases.
Main text
Microglia, the immune cells of the brain, form the first line of defense against neurodegenerative diseases and traumatic brain injuries. They maintain brain homeostasis, the stable condition necessary for survival, by acting like tiny vacuum cleaners—congregating at damaged areas, removing dead, infected, or injured brain cells and tidying up unnecessary synapses. However, microglia don’t originate in the brain, rather their precursor form travels there during development from their place of origin, another section of the embryo called the peripheral mesoderm. Many questions around this process, such as how the microglial precursors find their way, are currently unanswered. And, given how important these cells are for brain homeostasis in all animals, including humans, answering these questions could have a myriad of health benefits.
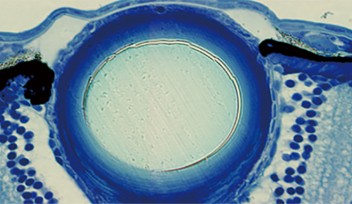
“Microglia have been implicated in several neurobiological diseases such as Alzheimer’s. Revealing how they work could shed light on these diseases,” stated Prof. Ichiro Masai who leads the Developmental Neurobiology Unit at the Okinawa Institute of Science and Technology Graduate University (OIST). “Microglia are found throughout the brain, but our most recent paper looks at how they colonize the retina—the neural tissue of the eye, which is one of the first brain regions to accommodate these cells.”
Reporting in eLife, Prof. Masai and former OIST PhD student Dr. Nishtha Ranawat revealed that, to successfully colonize the retina, microglia require two processes to have occurred—the blood vessels must have formed inside the eye, and neurogenesis, the process of neuron formation, must have begun within the retina.
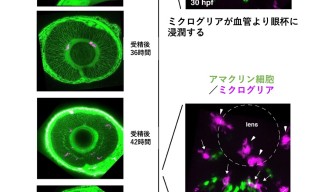
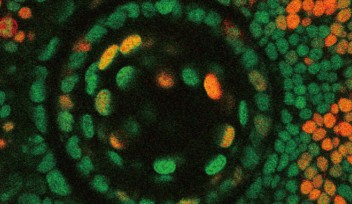
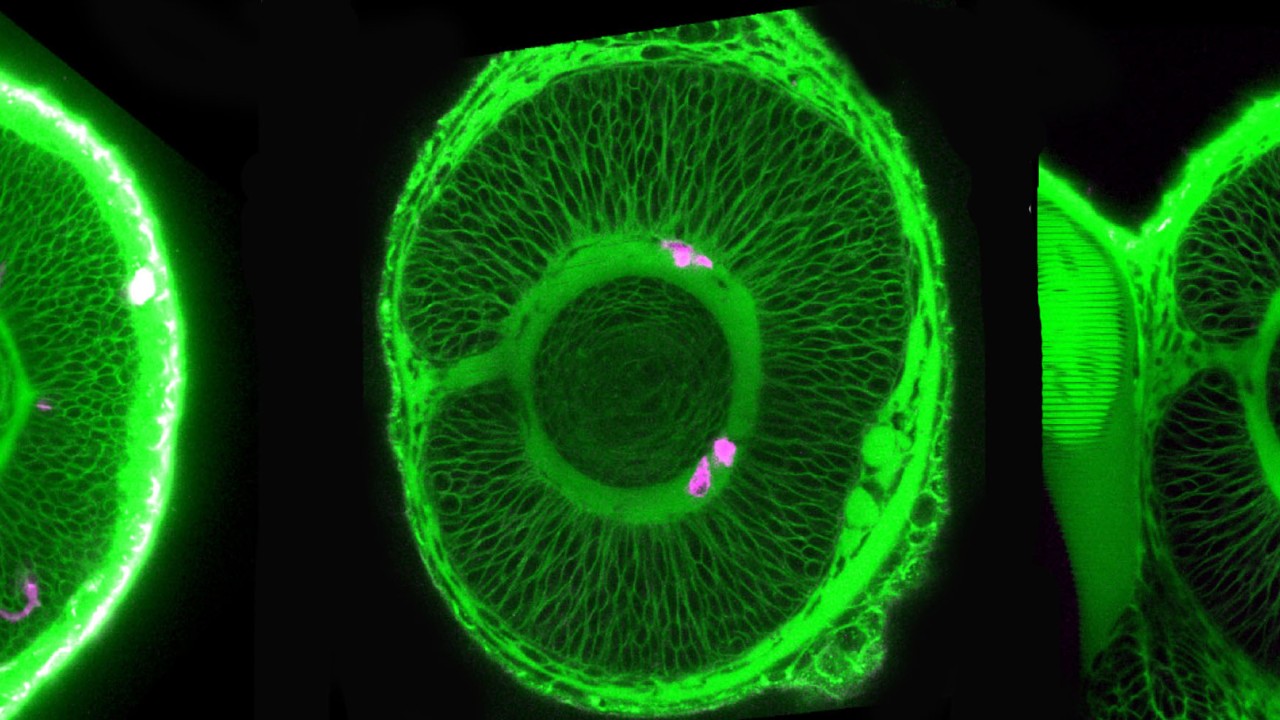
To uncover these requirements, the researchers imaged transparent embryonic zebrafish from 24 hours after fertilization to 60 hours after fertilization. They tagged the microglia precursors with fluorescence, which allowed them to be tracked. From their original place, these precursors first traveled to the yolk, and started migrating towards the various brain regions from there.
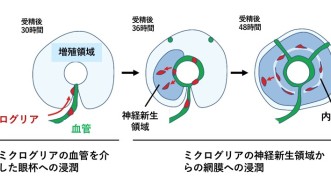
“The developing brain is very packed tissue,” explained Prof. Masai. “It’s hard to imagine how the microglia enter it and move around inside. But we found that they use the blood vessels as pathways to enter the eye.”
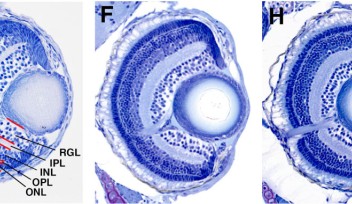
However, the journey doesn’t end there. Once the microglia arrive at the entrance of the retina, they remain associated with the blood vessels between the retina and the lens until another process begins—neurogenesis. Neurogenesis is the process of neuron formation. The researchers found that microglia could only infiltrate areas of the retina where neurogenesis was occurring. At around 60 hours after fertilization, the microglia were in place, spread throughout the retina.


To further determine that the blood vessels and neurogenesis were important components for microglia migration, the researchers hindered, in one experiment, the blood vessels from forming and, in another experiment, neurogenesis from occurring. In both cases, microglia were unable to enter the retina.
“This study highlights the microglia’s dependence on the developing retina blood vessels and their preference for differentiating neurons,” said Dr. Ranawat, who is the lead author of this study and now a Postdoctoral Scholar at Burke Neurological Institute in the USA. “In the future, this knowledge could lead to targeted microglia stem cell therapies for neurodegenerative diseases. The next step is to find the molecule dictating the interaction between the microglia and the blood vessels and neurons.”

Article information
Title: Mechanisms underlying microglial colonization of developing neural retina in zebrafish
Journal: eLife
Authors: Nishtha Ranawat and Ichiro Masai
DOI: 10.7554/eLife.70550
Date: 2021. 12. 07
Specialties
Research Unit
For press enquiries:
Press Inquiry Form