Cell division research explores questions at the core of life
In his laboratory at the Okinawa Institute of Science and Technology Graduate University (OIST), Professor Tomomi Kiyomitsu is researching one of the largest organelles – the mitotic spindle. If the word ‘spindle’ is ringing a bell, it might be because it was the object that Sleeping Beauty pricked her finger on, causing her to fall into an enchanted sleep in a well-known fairytale.
But Professor Kiyomitsu, who joined OIST in April 2020 to lead the Cell Division Dynamics Unit, is looking at a different kind of spindle – the one that exists within our cells. Although it’s one of the largest organelles, it’s still tiny – invisible to the naked eye – yet the role it plays is fundamentally important. The spindle is essential for maintaining genomic information and controlling the fate of daughter cells during cell division in all eukaryotes, including humans.
“When a cell divides in to two, all the genetic material, which is contained within chromosomes, must divide as well,” explained Professor Kiyomitsu. “The chromosomes always need to be equally distributed between the two daughter cells, and we know that the spindle is very important for this.”

Professor Kiyomitsu emphasized that there are so many unsolved, key questions in this field. “At its core, cell division provides the basis for all life,” he said. “It really is beautiful.”
The spindle in our cells
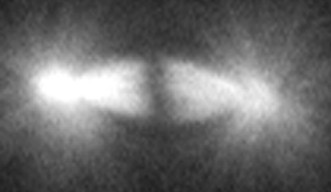
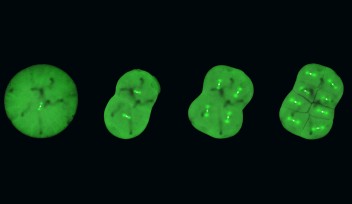
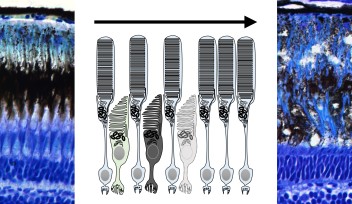
A spindle is made of a collection of microtubule fibers, which, during cell division, assemble into a diamond-shaped structure with two poles. Chromosomes are aligned in the center of the spindle and separated into two groups, one at each side of the spindle. This creates the foundation of the two daughter cells. The spindle also determines the site between the segregated chromosomes where the cell will split.

Professor Kiyomitsu is fascinated by how the spindle is properly assembled and positioned to allow for correct division. His research group has previously used human cells to identify a protein complex called the Dynein-Dynactin-NuMA (DDN) cluster, which is situated at the edge of the cell. They found that this cluster generated the forces to control the spindle positioning as the cell divides. Recently, they started using the cells of medaka – a species of fish – to visualize cell division during development and to understand how the complex and other mechanisms act in early embryonic division.
Focusing on the Ran pathway
To ensure that the spindle assembles correctly, the chromosomes must first generate signals that regulate hundreds of proteins, which, in turn, cause the microtubule fibers to cluster. Although scientists have previously determined that the DDN is important for spindle assembly and spindle positioning, they also know that there’s another player in the works – the Ran pathway, which governs at least some of the important proteins. But knowledge surrounding exactly how the Ran pathway controls each protein, and to what extent, is still very much in its infancy.
This year, Professor Kiyomitsu’s Unit had a paper published in Current Biology that looked at how essential the Ran pathway was to activate some of these proteins.
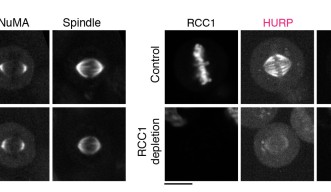
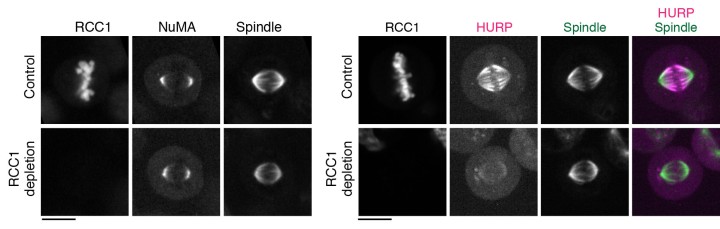
The group first focused on the activation of NuMA, a type of protein within the DDN complex, which is found at the poles of the spindle. Previous research has found that NuMA is vital for organizing the fibers at each pole. But how it is activated to start this organization at exactly the right moment is still a mystery. Professor Kiyomitsu theorized that it might have something to do with the Ran pathway, so the research group used OIST’s state-of-the-art imaging and resources to take a closer look.
They used a technique called auxin-inducible degron (AID), which allowed them to deplete any target protein within 30 minutes to determine exactly what role they played. Surprisingly, this revealed that the Ran pathway was not essential for activating NuMA. In fact, the Ran pathway was not involved in the assembly of any part of the spindle that wasn’t close to the chromosomes, which included the poles.
With this in mind, they decided to look at another two proteins– HURP and HSET – using the same technique. These two proteins are important for establishing the correct spindle length as the cell divides. The researchers found that the Ran pathway was essential for them to operate correctly. More specifically, the Ran pathway ensured that the two proteins were localized, or restricted, and remained within the area they needed to be.
Now that they’ve established this technique in human cells, Professor Kiyomitsu and his Unit plan to study other proteins in the DDN cluster and the spindle to determine their roles, not only in cultured human cells but also in mouse stem cells and the early embryos of Medaka. This research will continue to strengthen our understanding of the biology of cells – the building blocks of all living things.
“Mitosis has been studied for decades but, with these new techniques, we can look at a greater level of detail. The type of environment and funding available at OIST isn’t available elsewhere. This research really is at the forefront of the cell division field,” said Professor Kiyomitsu. “I plan to work with the resources and specialists within the Imaging Section at OIST to continue to visualize many new proteins and protein complexes and discover their functions within the spindle and the cell.”
Research Unit
For press enquiries:
Press Inquiry Form