Cracking Solar Cell Stability

Silicon dominates solar energy products — it is stable, cheap, and efficient at turning sunlight into electricity. Any new material taking on silicon must compete, and win, on those grounds. As a result of an international research collaboration, Shanghai Jiao Tong University, the Ecole Polytechnique Fédérale de Lausanne (EPFL), and the Okinawa Institute of Science and Technology Graduate University (OIST) have found a stable material that efficiently creates electricity — which could challenge silicon hegemony.
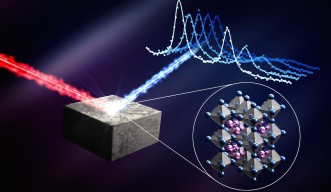
Writing in Science, the collaborating teams show how the material CsPbI₃ has been stabilized in a new configuration capable of reaching high conversion efficiencies. CsPbI₃ is an inorganic perovskite, a group of materials gaining popularity in the solar world due to their high efficiency and low cost. This configuration is noteworthy as stabilizing these materials has historically been a challenge.
“We are pleased with results suggesting that CsPbI₃ can compete with industry-leading materials,” says Professor Yabing Qi, head of OIST’s Energy Materials and Surface Sciences Unit, who led on the surface science aspect of the study.
“From this preliminary result we will now work on boosting the material’s stability — and commercial prospects.”

Energy level alignment
CsPbI₃ is often studied in its alpha phase, a well-known configuration of the crystal structure appropriately known as the dark phase because of its black color. This phase is particularly good at absorbing sunlight. Unfortunately, it is also unstable — and the structure rapidly degrades into a yellowish form, less able to absorb sunlight.
This study instead explored the crystal in its beta phase, a less well-known arrangement of the structure that is more stable than its alpha phase. While this structure is more stable, it shows relatively low power conversion efficiency.
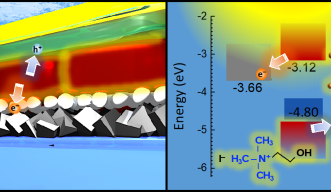
This low efficiency partly results from the cracks that often emerge in thin-film solar cells. These cracks induce the loss of electrons into adjacent layers in the solar cell — electrons that can no longer flow as electricity. The team treated the material with a choline iodide solution to heal these cracks, and this solution also optimized the interface between layers in the solar cell, known as energy level alignment.
“Electrons naturally flow to materials with lower potential energy for electrons, so it is important that the adjacent layers’ energy levels are similar to CsPbI₃,” says Dr. Luis K. Ono, a co-author from Professor Qi’s lab. “This synergy between layers results in fewer electrons being lost — and more electricity being generated.”

The OIST team, supported by the OIST Technology Development and Innovation Center, used ultraviolet photoemission spectroscopy to investigate the energy level alignment between CsPbI₃ and the adjacent layers. These data showed how electrons can then move freely through the different layers, generating electricity.
The results showed a low loss of electrons to adjacent layers following treatment with choline iodide —due to better energy level alignments between the layers. By repairing the cracks that naturally emerge, this treatment led to an increase in conversion efficiency from 15% to 18%.
While that leap may seem small, it brings CsPbI₃ into the realm of certified efficiency, the competitive values offered by rival solar materials. Although this early result is promising, inorganic perovskite is still lagging. For CsPbI₃ to truly compete with silicon, the team will next work on the trinity of factors allowing silicon’s reign to continue — stability, cost, and efficiency.
Header: "Photovoltaic Cell" by choctruffle is licensed under CC BY-NC-SA 2.0
Specialty
Research Unit
For press enquiries:
Press Inquiry Form