Chemists Create Intricate Designer Molecules
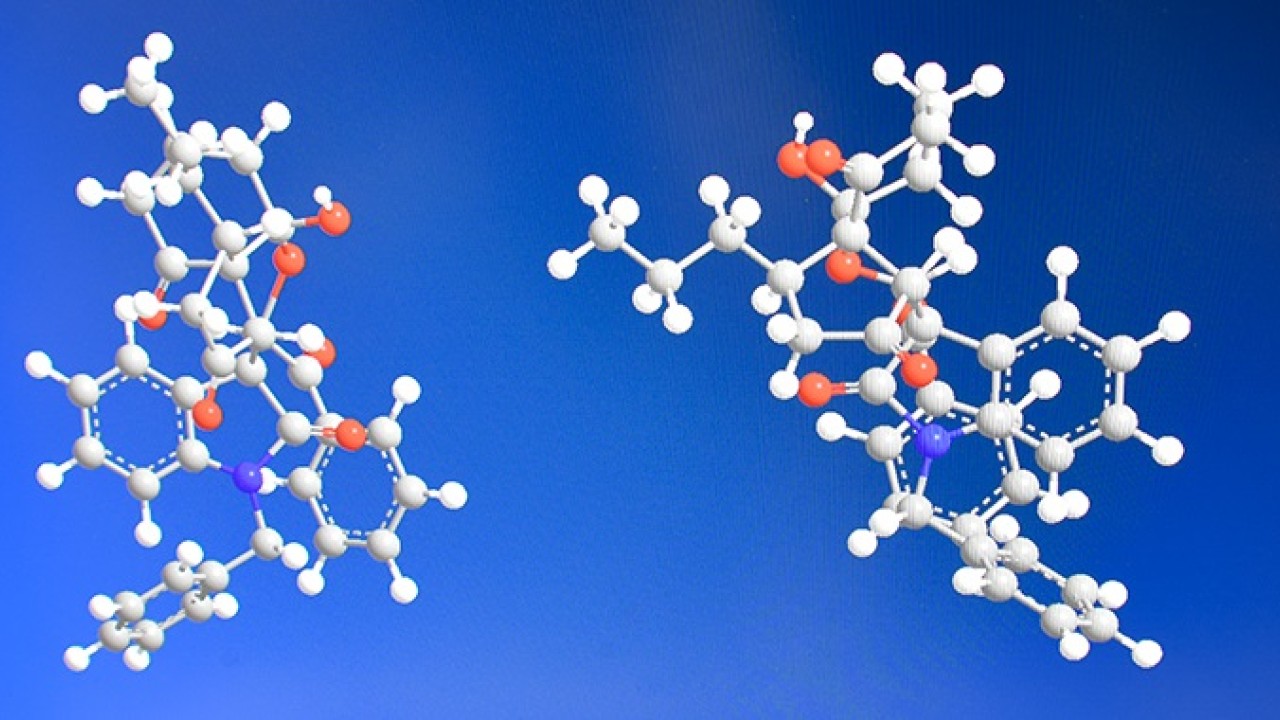
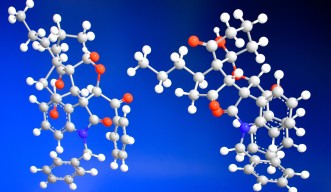
The natural world is full of complex molecules with important roles in living things. Such compounds can be useful as medicines — but they’re tricky to extract in large amounts. And while it is possible to create these molecules in the lab, the process is almost as tough. Scientists at the Okinawa Institute of Science and Technology Graduate University (OIST) report a simple strategy for synthesizing complex molecules called spiro pyran polycycles, or SPPs, which could be developed as pharmaceuticals.
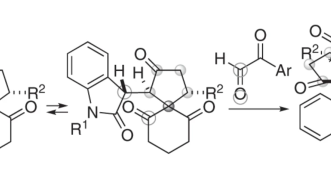
SPPs are a class of molecule named after their twisted shape (“spiro”), many carbon rings (“polycycle”), and oxygen molecules found on those carbon rings (“pyran”). Writing in Communications Chemistry (Springer Nature Publishing), the team details their recipe for creating a pure product of SPPs — a triumph in organic chemistry — in a single elegant step.
“Spiro polycycles are complicated, especially once we make them ‘pyran’ by switching a carbon for oxygen on some of the rings,” explains Dr. Sohail Muhammad, a postdoctoral scholar in OIST’s Chemistry and Chemical Bioengineering Unit. “But complexity is also part of their charm. It is satisfying to even create such molecules, and it might give them power as medicines.”

SPPs are tortuous molecules. Just as human hands are mirror images that cannot be superimposed, a molecule can also exist as its own mirror image. The non-superimposable points on a molecule are known as chiral centers — and SPPs have a whopping six of them, which highlights their complexity.
The OIST Chemistry and Chemical Bioengineering Unit previously produced a pure product of spiro polycycles, with rings on the molecule that only contain carbon atoms. They did this using a catalyst that only reacts with one configuration — one mirror image — of the molecule. This allowed them to synthesize one particular structure and obtain a pure product of it. What’s more, they did this in just two steps.
In the current study the researchers created enantiomerically pure forms of oxygen-containing SPPs, which are more difficult to synthesize than their all-carbon cousins. This is because replacing a carbon with an oxygen changes the distance between atoms, known as the bond length.

This shift in bond length does, however, give SPPs new qualities that could be exploited in medicine. Different configurations at chiral centers can lead to radically different functions, so getting a pure stereoisomer of the product is critical if the compounds are to be considered for applications. In this paper, the team produced such a product.
Obtaining a pure product is just the first step. While Sohail enjoys the challenge of intellectual pursuits, the team must now look beyond clever synthesis strategies and create molecules that might be useful in drug discovery. And knowing that SPPs resemble compounds found in nature provides a head start on where to look.

This paper is:
Muhammad Sohail and Fujie Tanaka
Dynamic stereoselective annulation via aldol-oxa-cyclization cascade reaction to afford spirooxindole pyran polycycles
Communications Chemistry 2019, 2, article number 73, doi:10.1038/s42004-019-0177-5
The URL of this paper: https://www.nature.com/articles/s42004-019-0177-5
Research Unit
For press enquiries:
Press Inquiry Form