Protein Scaffold

Right before a cell starts to divide to give birth to a daughter cell, its biochemical machinery unwinds the chromosomes and copies the millions of protein sequences comprising the cell’s DNA, which is packaged along the length of the each chromosomal strand. These copied sequences also need to be put back together before the two cells are pulled apart. Mistakes can lead to genetic defects or cancerous mutations in future cell generations.
Just like raising a building requires scaffolding be erected first, cells use biochemical scaffolding machinery to reassemble copied genomic fragments back into chromosomes. Researchers at the Okinawa Institute of Science and Technology Graduate University (OIST) have mapped the points along the genome where a scaffolding protein crucial to maintaining the genome's structure binds. The paper was published in Genes to Cells.
The protein complex, called condensin, is one of many that become active when cells replicate. Researchers in OIST's G0 Cell Unit used fission yeast to find the binding sites of this particular protein complex along chromosomal DNA. This type of yeast shares many important genes with us and also has one of the two known condensin complexes in humans. It also undergoes cell division by first creating copies of chromosomes like most human cells and has a very fast replication cycle, all of which facilitated the study.
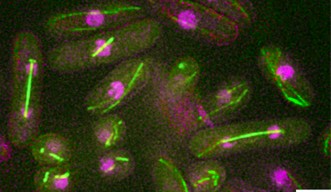
The OIST researchers found that the largest amount of condensin aggregates at the centromere, the central knot tying together the two replicated chromosomes. In a lot of cancerous cells, the centromere has an unnatural shape, which could be caused by a malfunction in the relevant cell’s scaffolding machinery.
Large amounts of condensin also accumulate at areas where RNA is created. In humans and all multicellular organisms, three different types of RNA producing enzymes control how genes are transcribed. Thus, condensin is crucial to passing on genes correctly.
Condensin also helps preserve the genome in challenging environments. OIST researchers bumped up the heat from 20 degrees to 36 degrees centigrade over 9 minutes, and found that condensin accumulated around heat-shock protein (Hsp) genes after replication. Hsp genes are a family of proteins produced by cells in stressful situations, ranging from high temperatures to ultraviolet light exposure to maintain genomic integrity.
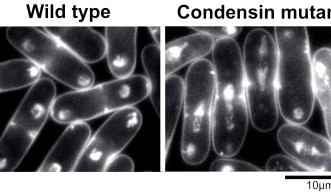
The researchers also engineered a yeast strain where a mutant condensin was produced by the cell when it went into figurative labor. In this mutated strain, there were massive errors in disentangling the separately copied chromosomes from the original. DNA content in the mutant cells increased and some of the resulting cell sizes were larger.
Larger cells need more energy to survive and condensin could be crucial to maintaining appropriate DNA content and cell sizes across cellular generations.
Extraneous structures like RNA and bound proteins are typically present along the length of chromosomes. Accommodating these extra structures into the daughter cell’s nucleus might be what increases the overall cell size.
“While these macromolecules are important for the parent cell, they pose hindrances during cell division to segregating the copied chromosomes to daughter cells properly,” said Dr. Norihiko Nakazawa, of OIST's G0 Cell Unit, the paper's first author.
The OIST researchers speculate that condensin is trimming the hedgerow of the chromosomes during the replication and dividing phase. They further speculate that these eliminated macromolecules might be reused by the cellular machinery of the daughter cell when necessary.
At this point, the relevant biochemical processes by which condensin works remain to be apprehended. The OIST study concentrates on only one type of condensin. Where the second type of condensin, which is present in humans and other multicellular organisms, binds during cell division is another future line of enquiry.
By Joykrit Mitra
For press inquiries contact media@oist.jp.
For press enquiries:
Press Inquiry Form