Interrogating Elusive Membrane Proteins
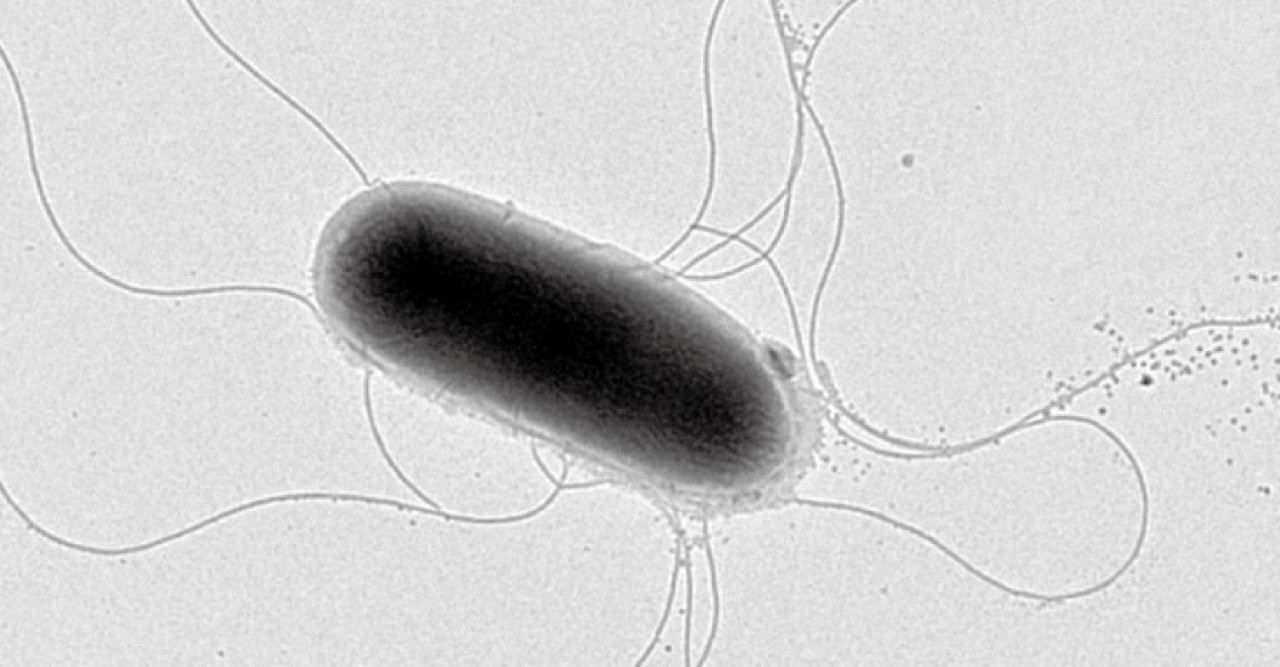
Despite recent decades’ dramatic advances in the tools used to study how living things work—increasingly powerful imaging methods, whole-genome sequencing, and access to vast amounts of data—cells still manage to keep some of their secrets. Among these are the workings of many of the notoriously difficult-to-characterize proteins embedded in the cells’ outer skin, or membrane. The Trans-Membrane Trafficking Unit, led by Fadel Samatey, is taking a sort of good cop-bad cop approach to one membrane protein complex, using two different means to grill it for information.
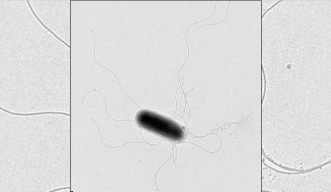
In order to discern a protein’s structure, researchers generally must get a relatively large amount of protein, purify it, and then coax it into forming a high-quality crystal that can be imaged by X-ray. But membrane proteins must be purified with a detergent, which hinders crystallization, explains Prof. Samatey. It’s also difficult to get a sufficient amount of a membrane protein in order to form a crystal. So tricky are membrane protein structures to study that most researchers steer clear of them, and those who dare to take them on can spend years on a project that never yields results. Nonetheless, Prof. Samatey has long been drawn to these enigmatic proteins. “I like challenges,” he explains. His unit studies a complex of membrane proteins responsible for building the corkscrew-like flagellum that propels some types of bacteria. Since this structure is very similar to the complex that builds the needle some bacteria use to inject toxins into host cells, learning more about it could help prevent or treat infection by pathogenic bacteria such as E. coli and Salmonella.
While some members of the unit are using the purify-and-crystallize approach to characterizing the proteins, Researcher Clive Barker is taking a different tack. Since the flagellum-building proteins are so similar between species of bacteria, he swaps the genes for one of the flagellum proteins from one species of bacteria with those from another species, then grows the bacteria in a soft agar that lets him see whether they’re moving. The bacteria remain still for a few generations, indicating that they lack functioning flagella, but then traces of movement start to appear in the agar, showing that some of the colonies have adapted to their new proteins. Dr. Barker sequences the genomes of these bacteria to find out where those adaptations occurred, as this provides clues about which proteins the swapped protein interacts with—and thus how, exactly, it works. The approach has already yielded some surprising results, but to really make sense of them, he’ll need the proteins’ structures, Dr. Barker says. By sending in both the good cop and the bad cop, the unit members are set to begin to crack their tough subjects.
Specialties
For press enquiries:
Press Inquiry Form