Marine Climate Change Unit
Professor Timothy Ravasi
Abstract
Our unit aims to understand how coral reef fish adapt to environmental changes such as climate change, heatwaves, overfishing and urbanization. Earth's oceans are warming and acidifying due to increasing anthropogenic CO2 production, and extreme events such as marine heatwaves are increasing in frequency, duration, and magnitude. Coral reef fish are especially vulnerable to spikes in temperature because they are already adapted to living close to their thermal limits. Determining which species can adapt to rapid environmental change, and how they will do so, is critical for predicting the ecological effects of climate change and their impact on the world economy.
In our laboratory research, we investigate how short-term as well as heritable changes in gene expression in multiple fish species are affected by increases in sea level temperature and acidity. We study animals that are descended from wild breeding pairs and have been reared in our aquariums over several generations. Using a unique device called the Heatwaves Simulator aquarium system based at OIST’s Marine Science Station, we expose fish to specific conditions, including the temperature and acidity level predicted to occur in seawater by the end of the century. We combine these manipulations with a range of advanced genomic technologies to identify the cellular mechanisms that allow coral reef fish communities in Okinawa and around the world to acclimate and adapt to changing conditions.
1. Staff
- Tae Woo Ryu, Research Unit Group Leader
- Roger Huerlimann, Postdoctoral Scholar
- Shannon McMahon, Postdoctoral Scholar
- Nicolas Dierckxsens, OIST Interdisciplinary Postdoctoral Scholar (50/50 with Nick Luscombe Unit)
- Erina Kawai, Research Unit Scientific Diver
- Jeffrey Jolly, Research Unit Technician
- Chengze Li, Research Unit Technician
- Gelyn Bourguignon, Fish Husbandry Technician
- Michael Izumiyama, Graduate Student
- Billy Moore, Graduate Student
- Callum Hudson, Graduate Student
- Ayşe Haruka Oshima Açıkbaş, Graduate Student
- Kaisar Dauyey, Special Research Student
- Johanna Linnea Johansson, Research Intern
- Yoko Shintani, Research Unit Administrator
2. Collaborations
2.1 Anemonefish development and genomics
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor Vincent Laudet, OIST, Japan
2.2 Coral Reef fish adaptation to marine heatwaves
- Type of collaboration: Scientific Collaboration
- Researchers:
- Professor Philip Munday, James Cook University, Australia
- Professor Jennifer Donelson, James Cook University, Australia
- Professor Jacob Johansen, University of Hawaii
2.3 Coral Reef fish adaptation to Ocean Acidification
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor Celia Schunter, The University of Hong Kong, HongKong
2.4 Coral Reef fish genomics
- Type of collaboration: Scientific Collaboration
- Researchers:
- Deputy Director Piero Carninci, RIKEN Center for Integrative Medical Sciences, Japan
- Professor Jesper Tegnér, KAUST, Saudi Arabia
- Professor Valerio Orlando, KAUST, Saudi Arabia
2.5 Coral Reef fish Physiology
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor Martin Grossel, University of Miami, USA
2.6 Impact of coastal development on coral reef ecosystems
- Type of collaboration: Scientific Collaboration
- Researchers:
- Professor James Davis Reimer, University of Ryukyus, Japan
- Professor Noriyuki Satoh, OIST, Japan
2.7 Natural analogues of climate stressors
- Type of collaboration: Scientific Collaboration
- Researcher:
- Dr. Riccardo Rodolfo-Metalpha, UMR ENTROPIE, Nouvelle Calédonie
2.8 Natural Volcanic CO2 Analogues
- Type of collaboration: Scientific Collaboration
- Researchers:
- Professor Ivan Nagelkerken, The University of Adelaide, Australia
- Professor Sylvain Agostini, University of Tsukuba, Japan
2.9 Okinawa Coral spawning and development
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor David J. Miller, James Cook University, Australia
2.10 Sharks physiology under climate stressors
- Type of collaboration: Scientific Collaboration
- Researchers:
- Professor Jodie L. Rummer, James Cook University, Australia
- Professor Rui Rosa, Faculdade de Ciências da Universidade de Lisboa, Portugal
2.11 Transgenerational acclimation
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor Jennifer Donelson, James Cook University, Australia
3. Activities and Findings
3.1 Design and Construction of the OIST Heatwaves Simulator aquariums system.
This year our unit in collaboration with Luxaqua, we designed and constructed the OIST Heatwaves Simulator, a unique aquariums system that will allows us and our collaborators to recreate a future environment including the simulation of marine heatwaves at the OIST Marine Science Station.
The OIST Heatwaves Simulator consist of three separate tank systems (Figure 1); 1) 10, 100L outdoor breeding pair tanks that will be used to house brood stock breeding pairs, 2) 10, 75L larval rearing tanks, that from 2022 onwards (F2, F3) will be used in the larval rearing stage of this multi-generational experiment, 3) 144, 55L tanks that will house experimental juvenile fish in different treatments. Temperature within these experimental tanks is controlled by four inline heaters, that allow five different temperature treatments to be specified in each individual tank. Temperature is controlled and monitored by automated software and associated temperature probes. Software also has remote monitoring and control capabilities.
This system is unique in the world and it will help to incentive collaborators from Japan and overseas to come to OIST and work with us at the marine station.
3.2 Coral Reef fish Physiology and Metabolic Performance
To assess the effects of the different heatwave treatments on coral reef fish’s metabolic rates, in collaboration with Loligo Systems, we set up at the OIST Marine Station a 170ml mini-swim tunnel (Figure 1). Water flow within the swim tunnel is generated by a propeller, and laminar flow is created by a series of flow straightening plastic honeycomb meshes. The velocity of water within the tunnel is controlled by Loligo Systems AutoResp software and is calibrated using Loligo Systems fluorescent PE microspheres and the associated Digital Particle Tracking Velocity software. An oxygen dipping probe is inserted into the swim tunnel, allowing for continuous measurements of oxygen concentration within the tunnel. A pump can be used to flush the swim tunnel with fresh seawater from the waterbath if required. The swim tunnel is submerged within a temperature controlled waterbath allowing the temperature within the swim tunnel to be manipulated. Flushing of the swim-tunnel and resting chambers, monitoring of oxygen concentrations and temperature, and calculations of oxygen consumption rates are all completed automatically by Loligo Systems AutoResp software.
3.3 Amphiprion ocellaris Spawning and Larval Rearing
In order to conduct the planned long-term multi-generational experiment detailed above, we first needed to ensure that wild-caught A.ocellaris from Okinawan coral reef systems would spawn within aquaria. To test this, one breeding pair of A.ocellaris was acquired from local fishermen in September 2019. This pair was placed within a 200L aquaria at OIST Marine Science Station with a sea anemone (Heteractis magnifica) and multiple rocks.
On the 31st of May 2020 eggs were observed within the breeding pair tank (Figure 1). The development of eggs was tracked using visual observation and comparisons to know stages of embryonic development. On the 7th of June, eggs were transferred to a larval rearing tank for hatching (Figure 3) and larval A.ocellaris were present in the tank on the 8th of June.
The successful spawning and larval rearing program of 2020 allowed us to develop for the first time at OIST Marine Science Station a larval rearing protocol.
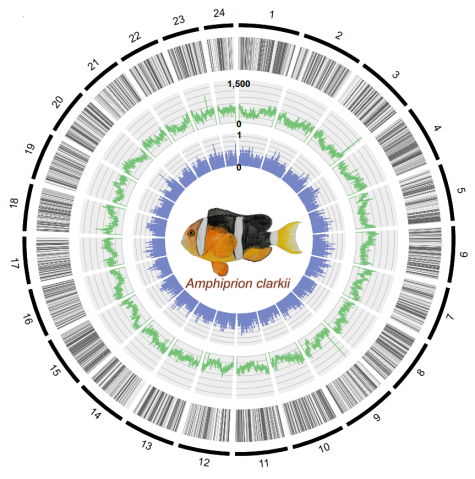
3.4 The Sequencing of Amphiprion ocellaris, Amphiprion clarkii and Epinephelus malabaricus genomes.
Our unit sequenced the genomes of three coral reef fish from the water of Okinawa island, the Amphiprion ocellaris, Amphiprion clarkii and Epinephelus malabaricus (Table 1). All these genomes have been sequenced using PacBio long-reads sequencing combined with Illumina gDNA and transcriptome sequencing and binned into chromosomes using Hi-Seq contact map technology.
These genomes are among the most completed fish genomes ever sequenced and are available free for the entire community.
3.5 Natural Analogues of Future Climate
Unique sites which are natural analogues of future ocean have a community that already exists under this naturally occurring extreme environment and can be utilized as a natural laboratory. These sites are subjected to daily changes in ocean chemistry, have dynamic interactions between habitat, predator, and prey, and have existed in long timescales. Inhabitants may have also adapted to these extreme conditions through generations. Although conditions at each extreme site vary and are not perfect simulations of future oceans, they can provide a window into how species may respond to climate change.
Our unit successful performed field expeditions at the following natural analogues.
Bourake, New Caledonia
In the spring of 2020, with the collaboration of the IRD and several other universities, we conducted a two-week expedition to Bourake, New Caledonia, where we collected four species of fishes (Dascyllus aruanus, Zoramia leptacantha, Chaetodon lunulatus, Pomacentrus molluscensis) from future and control sites.