Blind Zebrafish Shed Light on Human Retinal Diseases

Featured on the cover of Developmental Cell, a paper by OIST researchers revealed the mechanistic link between photoreceptor cell degeneration and defects in protein transport.
Like the shipment of goods within a country or around the world, the system that transports proteins within a cell must function properly in order to ensure the cell’s health. Protein transport is carried out by vesicles – the semi-trucks, trains and container ships of the cellular world. These tiny bubbles transport the proteins within them by budding off and subsequently fusing with different membranes within the cell. When the protein transport system breaks down due to defects, the cell undergoes apoptosis, or programed cell death.
Apoptosis in photoreceptor cells in the eye is associated with a disease called retinitis pigmentosa, which eventually leads to blindness. While previous studies have elucidated a connection between defects in protein transport and photoreceptor apoptosis, the mechanism behind this link remained elusive.
In a paper featured on the cover of the May 28, 2013 issue of Developmental Cell, researchers in the Developmental Neurobiology Unit at OIST, led by Professor Ichiro Masai, revealed the mechanism underlying the link between the photoreceptor cell degeneration and defects in protein transport within these cells.
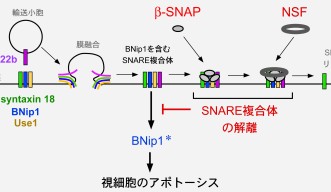
The process by which a vesicle fuses to a membrane within a cell is moderated by a group of proteins called the SNARE complex. When one SNARE protein on the surface of a vesicle meets the three other SNARE proteins on the target membrane it triggers the vesicle fusion process. Two other proteins, β-SNAP and NSF, then disassemble the SNARE complex to prepare for another round of fusion.
Using zebrafish with a nonfunctional β-SNAP protein as a model organism, the researchers found that when the SNARE complex failed to disassemble, one of the SNARE proteins on the target membrane, BNip1, activated the apoptosis of the photoreceptor cell. However, when β-SNAP is functioning properly it prevents BNip1 from initiating programed cell death. In this way, the dual function of BNip1 as a member of the SNARE complex and a protein that can initiate photoreceptor apoptosis provides the link between these two processes.
“BNip1 is like the big red abort button in the photoreceptor cell,” says Dr. Yuko Nishiwaki, a researcher in the Unit and lead author on the paper. “By uncovering what can cause the death of photoreceptor cells, we’re now better equipped to find cures for forms of blindness and vision impairment like retinitis pigmentosa.”
Research Unit
For press enquiries:
Press Inquiry Form