Iron catalyst could make important chemical reactions cheaper and more eco-friendly
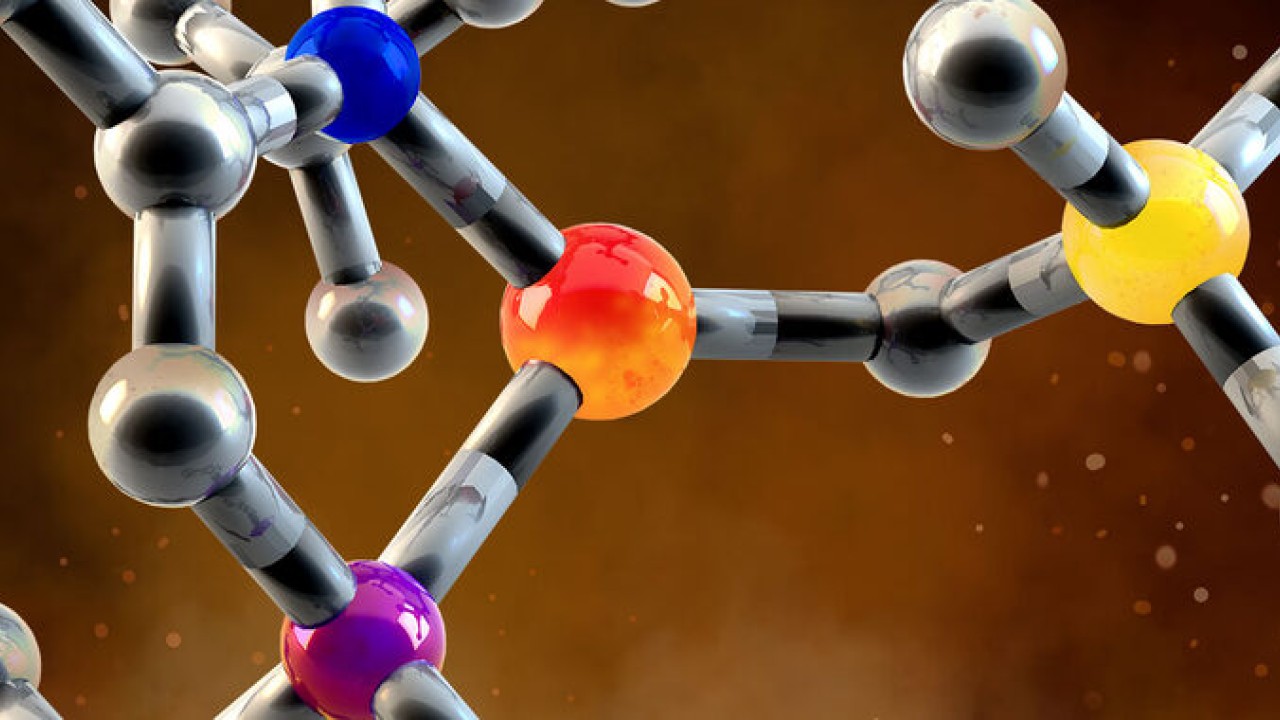
A catalyst is a key ingredient for many chemical reactions. It facilitates a molecule to change into another molecule, without being changed itself, meaning it can be used multiple times. However, many catalysts are made of precious metals, making them expensive and potentially harmful to the environment. Now, researchers have designed a catalyst made of a much more abundant metal—iron—to facilitate an important chemical reaction—the olefin metathesis reaction. Their work was published recently in Nature Catalysis.
“The olefin metathesis reaction is among the most widely applicable catalytic reactions for carbon-carbon double bond formation,” explained Satoshi Takebayashi, a researcher at the Okinawa Instiute of Science and Technology Graduate University (OIST) who was involved in the work. “Carbon-carbon double bonds are an important bond found in many chemical products.”
Olefins are a class of compounds with carbon-carbon double bonds. The olefin metathesis reaction produces new carbon-carbon double bonds by swapping the carbon atoms in olefins. The catalyst facilitates this swapping by breaking the original double bonds and causing new ones to form.
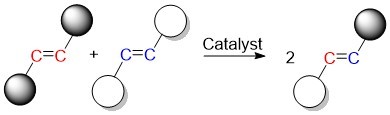
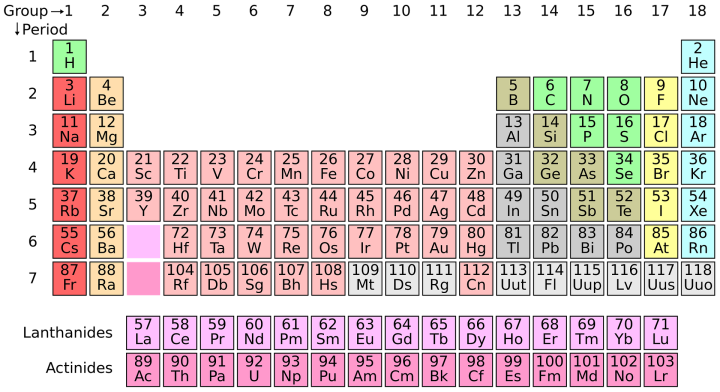
Currently, one of the most popular catalysts for this reaction is made from the precious metal, ruthenium. The aim of this study was to facilitate the reaction using a catalyst made with a much more abundant metal, iron, thus making the whole process cheaper and more environmentally friendly. This has been a long-sought goal in the scientific community as ruthenium and iron are in the same group on the periodic table and so are expected to have similar properties.
For this study, the researchers designed a new iron complex and demonstrated that it could be used as a catalyst in the olefin metathesis reaction. They showed that it worked by creating a polymer—a long chain molecule made of many smaller chemical units.
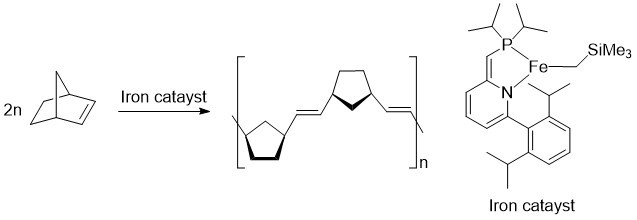
Despite the success of this research, Takebayashi highlighted that the state-of-the-art ruthenium-based catalysts are still much more applicable than the newly created iron-based ones. The iron-catalyst is unstable and less active when exposed to air and moisture. These limitations need to be fixed before the iron-catalyst can replace the ruthenium one.
“This study can be useful to other researchers in the field,” concluded Takebayashi. “I hope that iron-based catalysts can be developed further using this knowledge.”
To learn more about this research, please see this blog post written for Chemistry Community by Dr. Takabayashi.
Article Information
Research Unit
For press enquiries:
Press Inquiry Form