Feel the force: new “smart” polymer glows brighter when stretched

Scientists from the Okinawa Institute of Science and Technology Graduate University (OIST) have created a stress-detecting “smart” polymer that shines brighter when stretched. Researchers hope to use the new polymer to measure the performance of synthetic polymers and track the wear and tear on materials used in engineering and construction industries.
The scientists developed this polymer by incorporating copper complexes – structures formed by linking copper atoms to organic (carbon-containing) molecules – into a polymer called polybutylacrylate, which is made from a chemical used to synthesize acrylic paints, adhesives and sealants.
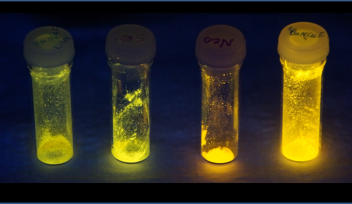
The copper complexes, which link the polybutylacrylate chains together, naturally glow when exposed to ultraviolet light – a property known as photoluminescence. But when the polymer is stretched, the copper complexes emit light at a greater intensity, leading to a brighter glow. The copper complexes therefore act as mechanophores – compounds which undergo a change when triggered by a mechanical force.

Most mechanophores are made not from metals such as copper, but from organic compounds, which change color or emit light when mechanical stress breaks a weak chemical bond. But mechanophores that use this bond-breaking mechanism have severe limitations.
“A relatively large force is required to break the chemical bond, so the mechanophore is not sensitive to small amounts of stress,” said Dr Ayumu Karimata, first author of the study and a postdoctoral scholar from the OIST Coordination Chemistry and Catalysis (CCC) Unit, led by Professor Julia Khusnutdinova. “Also, the process of breaking the bond is often irreversible and so these stress sensors can only be used once.”
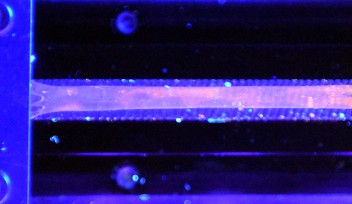
In contrast, the new copper mechanophores developed by the CCC unit are sensitive to much smaller stresses and can respond quickly and reversibly. In the study, published in Chemical Communications, the scientists reported that the polymer film immediately brightened and dimmed in response to being stretched and released.
Shining a light on the mechanism
Photoluminescent compounds, such as these copper complexes, have long been a topic of interest for the CCC unit. Prior to making the polymer, the researchers synthesized isolated copper complexes of varying size.

The team found that the copper complexes were very dynamic, continuously distorting in shape. But as they increased in size, the copper complexes became less flexible and glowed brighter. The CCC unit believes that the larger, less flexible complexes release light more efficiently because their motion is restricted, and they therefore lose less energy as heat.

The researchers realized they could exploit the relationship between the flexibility of the copper complexes and brightness to create a stress-detecting polymer.
“When the copper complexes are incorporated into the polymer as cross-links, the act of stretching the polymer also reduces the flexibility of the molecules,” explained Karimata. “This causes the copper complexes to luminesce more efficiently with greater intensity.”
Although still a long way off, Dr Karimata hopes that the acrylic polymer could eventually be adapted to create a stress-sensing acrylic paint. This could have valuable applications as a coating for different structures, such as bridges or the frames of cars and aircraft.
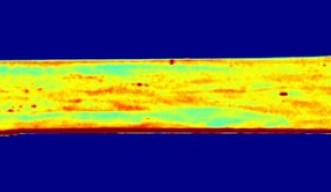
“As we can see even from the direct visualization of the polymer, stress is applied across a material in a non-uniform way,” said Karimata. “A stress-sensing paint would allow hotspots of stress on a material to be detected and could help prevent a structure from failing.”

Header image reproduced by permission of Dr Julia Khusnutdinova and The Royal Society of Chemistry from Chem. Commun., 2020, 56, 50.
Specialty
Research Unit
For press enquiries:
Press Inquiry Form